Stay up-to-date and subscribe to our newsletter
Sign up to stay informed and receive information on healthcare innovation, straight to your inbox
During the COVID-19 pandemic, BioTel Research is intensely focused on making sure you have the cardiac safety testing support you need. Our team is working tirelessly to deploy remote monitoring services that minimize person-to-person contact, while maintaining vital health information flow. We are also ensuring the continuation of current trials, as well as providing support for the rapid development of new vaccines and therapies for the novel coronaviris.
Cardiac Monitoring Challenges During the Pandemic
Advantages of Remote Cardiac Monitoring
 MCOT Used in COVID-19 Monitoring Nationwide
MCOT Used in COVID-19 Monitoring Nationwide
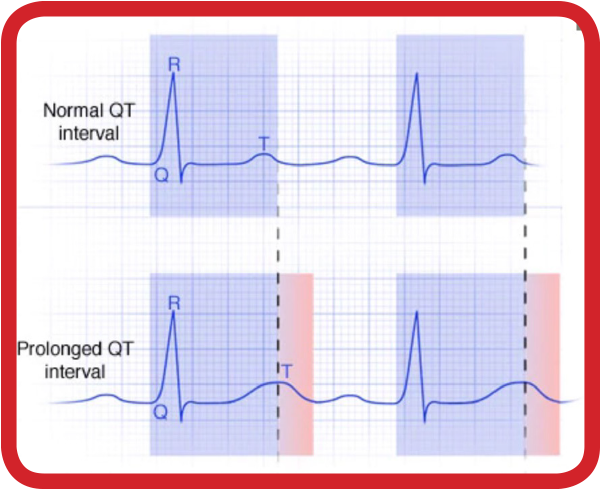
Cardiac Risks, Including QT Prolongation, are Associated with Some Experimental COVID-19 Therapies
MCOT: FDA-Approved to Measure, Analyze, and Report QT Intervals

MCOT™ Patch (Mobile Cardiac Outpatient Telemetry):
Superior Arrhythmia Detection in a Convenient, Wearable Patch
Chosen by Professionals During the COVID-19 Pandemic — for Clinical Trials and Clinical Practice
* Based on MIT-BIH (Massachusetts Institute of Technology-Beth Israel Hospital) Atrial Fibrillation Database testing of ≥30-second AF episodes. (FDA 510k submission)

In response to COVID-19, Northwell Health chose MCOT™ (Mobile Cardiac Outpatient Telemetry) to more closely monitor cardiac safety for patients receiving hydroxychloroquine and azithromycin.
Key Points:
Reference: 1. Gabriels J, et al. Inpatient Use of Mobile Continuous Telemetry for COVID-19 Patients Treated with Hydroxychloroquine and Azithromycin. Heart Rhythm Case Reports. Accesed on the Web. 2 April 2020, https://www.heartrhythmcasereports.com/article/S2214-0271(20)30058-0/fulltext